PLM-Enabled Quality Compliance for Medical Device Part 2 – Design Controls
With today’s quality compliance challenges, life science and medical device companies can use product lifecycle management (PLM) as a backbone to support compliance, while enabling continued innovation. PLM connects performance data and enables FDA compliance in six key functions: design controls, document and change controls, corrective and preventative action, material controls, production and process controls and equipment and facility controls.
Let’s dive into design controls to examine the FDA requirements and how life science and medical device companies can leverage PLM to enable visibility across all projects and the product design activities.
Newton’s first law states that for every action, there is an equal and opposite reaction. In product development, I’d say for every input, there is always an output.
However, the output may differ substantially from the required input and deviate from the proper functions of the product. Much of the deviation is related to lack of documentation to support and justify the output. Procedures must be established to ensure that the design requirements relating to the product are appropriate and address the intended use of the device, including the needs of the user and patient.
The FDA requires that all medical manufacturers have design controls in place to establish and maintain a reference for design and development1. Management teams must conduct periodic reviews to the product development process to track verification and validation of the design inputs, and they must ensure that the design inputs are adequately realized in the actual outputs. In addition, if there are post-release changes or events that impacted the original design, the manufacturer must document and maintain a record of these incidents and the changes that took place.
Most companies face a communication disconnect between product developers, manufacturing, quality managers and business divisions within the company. As a result, managers struggle to decipher product performance and its impact on the business strategy. Another common challenge is the lack of synchronization across enterprise systems. Working in silos creates a chaotic environment with no cross-functional collaboration, resulting in a straggle of performance.
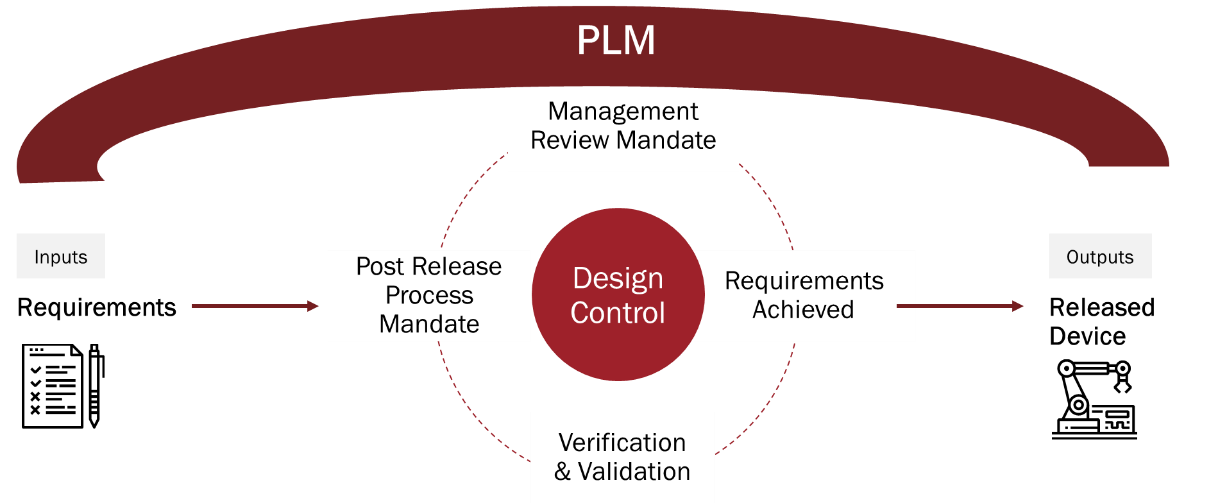
Medical device manufacturers can leverage a PLM platform to electronically track and improve design controls, including (but not limited to) engineering drawings, bill of materials, product formulas and SOPs. A PLM system can also ensure traceability between the design inputs and design outputs, and it can confirm that quality issues and design inputs are correlated in order to resolve any downstream problems without losing connectivity to the original requirements.
Consequently, PLM can reduce product time-to-market by sharing and maintaining control of the design data in a collaborative environment. Companies can distribute data as needed, and ultimately deliver a safe and practical product to patients.
Ultimately, PLM provides visibility across all projects and the product design activities, so that the product output is equal to the product input.
1 FDA 21 CFR Part 820.30 and ISO 13485 section 7.3
