PLM-Enabled Quality Compliance for Medical Device Part 1 – PLM at the Core
Life science and medical device companies are faced with intensifying regulatory scrutiny and growing product quality demands. The challenge is to navigate the labyrinth of quality compliance while remaining competitive and continuing to innovate. This blog series will focus on the systems that enable compliance, starting with the core system - product lifecycle management (PLM).
The FDA method for evaluating compliance is the Quality System Inspection Technique (QSIT). QSIT allows the FDA to target the six major quality functions and systems that life science and medical device companies must have in place. An organization’s PLM system, as it relates to each of the six quality functions, provides either a direct solution or acts as a supporting interface to ERP or MES systems.
Beyond compliance, there’s tremendous value in sharing knowledge and data across the activities in these six key quality system functions:
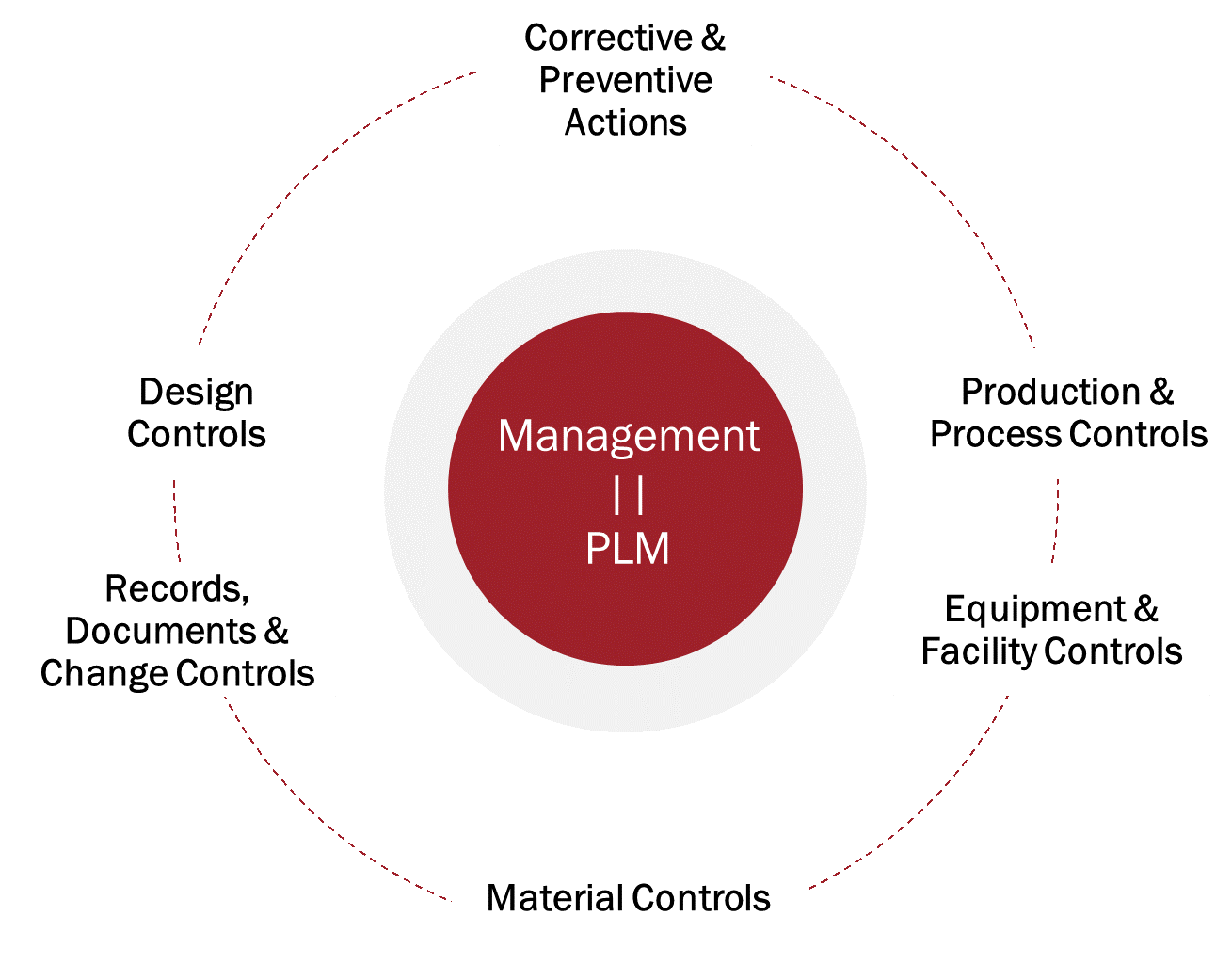
- Design Controls
- Records, Documents & Change Controls
- Corrective & Preventative Actions
- Material Controls
- Equipment & Facility Controls
-
Production & Process Controls
PLM is the digital backbone model of an organization, connecting performance data across the value chain, monitoring real time quality management, consolidating systems for data continuity, stimulating enterprise wide collaboration and enabling a product centric model.
In support of core business processes, it’s also important to recognize the benefits that PLM will provide across the entire enterprise. Business models constantly evolve, and digital technologies enable new and flexible options for maintaining both a competitive advantage and global quality regulatory compliance.
Leading life science and medical device companies streamline compliance by integrating constantly-changing regulations into product development and across the product design process. Whether the focus is project management, market requirements, quality, traceability or reporting, it is vital for organizations to implement global PLM to synchronize business process, product quality and maintain system integrity.