PLM-Enabled Quality Compliance for Medical Device Part 7 – Production & Process Controls
With today’s quality compliance challenges, life science and medical device companies can use product lifecycle management (PLM) as a backbone to support compliance, while enabling continued innovation. PLM connects performance data and enables FDA compliance in six key functions: design controls, document and change controls, corrective and preventative action, materials controls, production and process controls and equipment and facility controls.
This blog will demonstrate how the collaborative environment between PLM, ERP and MES will help companies ensure that a product conforms to its specifications*, by establishing and maintaining the sixth major quality function, Production and Process.
Medical device manufacturers are required to:
- be able to identify any quality events related to production processes
- manage how quality events and design changes can impact the manufacturing of the product
- determine and manage all risks associated with the production process changes in order to explain any necessary re-qualification decisions
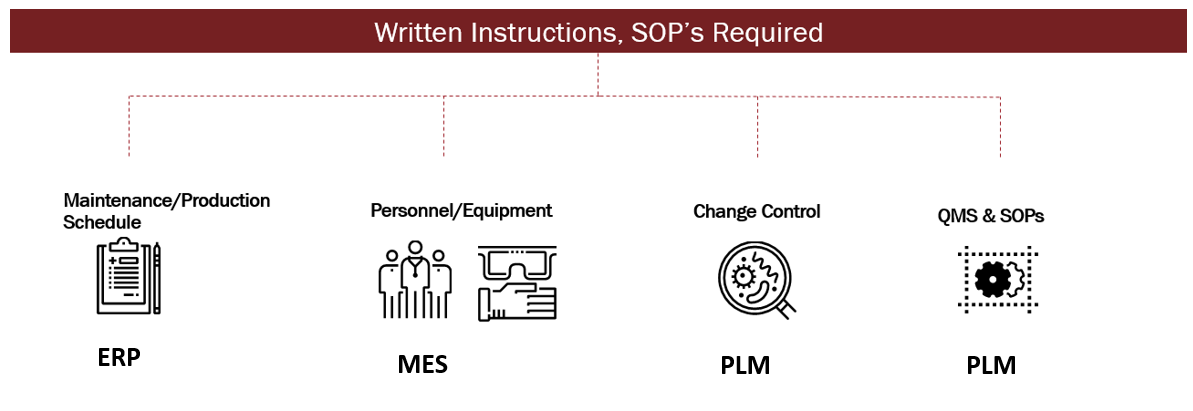
The figure above describes how the links between the PLM, ERP and MES systems provide a comprehensive approach to a successful Quality Management System (QMS) that complies with the FDA requirements to develop thorough processes and procedures, and meets Current Good Manufacturing Practice regulations (cGMP).*
So, what is the value beyond compliance?
Well, it’s an evolution (not a revolution) because mandating compliance is not a guarantee of a products’ medical quality. When mapping the entire process with the help of PLM, the organization is able to virtually identify and control all criteria across the process lifecycle without re-inventing the wheel. Documenting all steps in the process, as well as creating instructions, SOPs and training regimens, will eliminate any “language barrier” between all sectors of the enterprise and global regulatory bodies. This ultimately results in better:
- Risk management
- Timely and accurate response to audits
- Quality process standards
- Innovative and quality product standards
To sum it all up, a PLM system acts as a single repository for product quality, business performance, production, supply chain and compliance integrity, and also connects the past, present and future of the product development history of an organization.
* FDA 21 CFR Part 820.70 and GMP 11.1