PLM-Enabled Quality Compliance for Medical Device Part 5 – Material Controls
With today’s quality compliance challenges, life science and medical device companies can use product lifecycle management (PLM) as a backbone to support compliance, while enabling continued innovation. PLM connects performance data and enables FDA compliance in six key functions: design controls, document and change controls, corrective and preventative action, material controls, production and process controls and equipment and facility controls.
This blog will expound on how essential it is to maintain accurate data flow and integrated collaboration across PLM-ERP-MES as it pertains to the fourth major quality function: Material Controls.
A fundamental challenge in the life science and medical device industry is to get the right product, at the right time with the right price to the physicians and patients. The use of technology has become a standard practice in today’s competitive market, which leads us to the conclusion that Enterprise Resource Planning (ERP) and Manufacturing Execution Systems (MES) are essential, but not sufficient to sustain a competitive advantage, deliver a quality product to the patients, and operate in an effective and efficient environment. Additionally, and from a regulatory compliance prospective1, companies are required to maintain a uniform system that tracks materials and associated suppliers in production, across the entire enterprise, in order to verify that the quality of the materials used coincided with the released product and its design specifications.
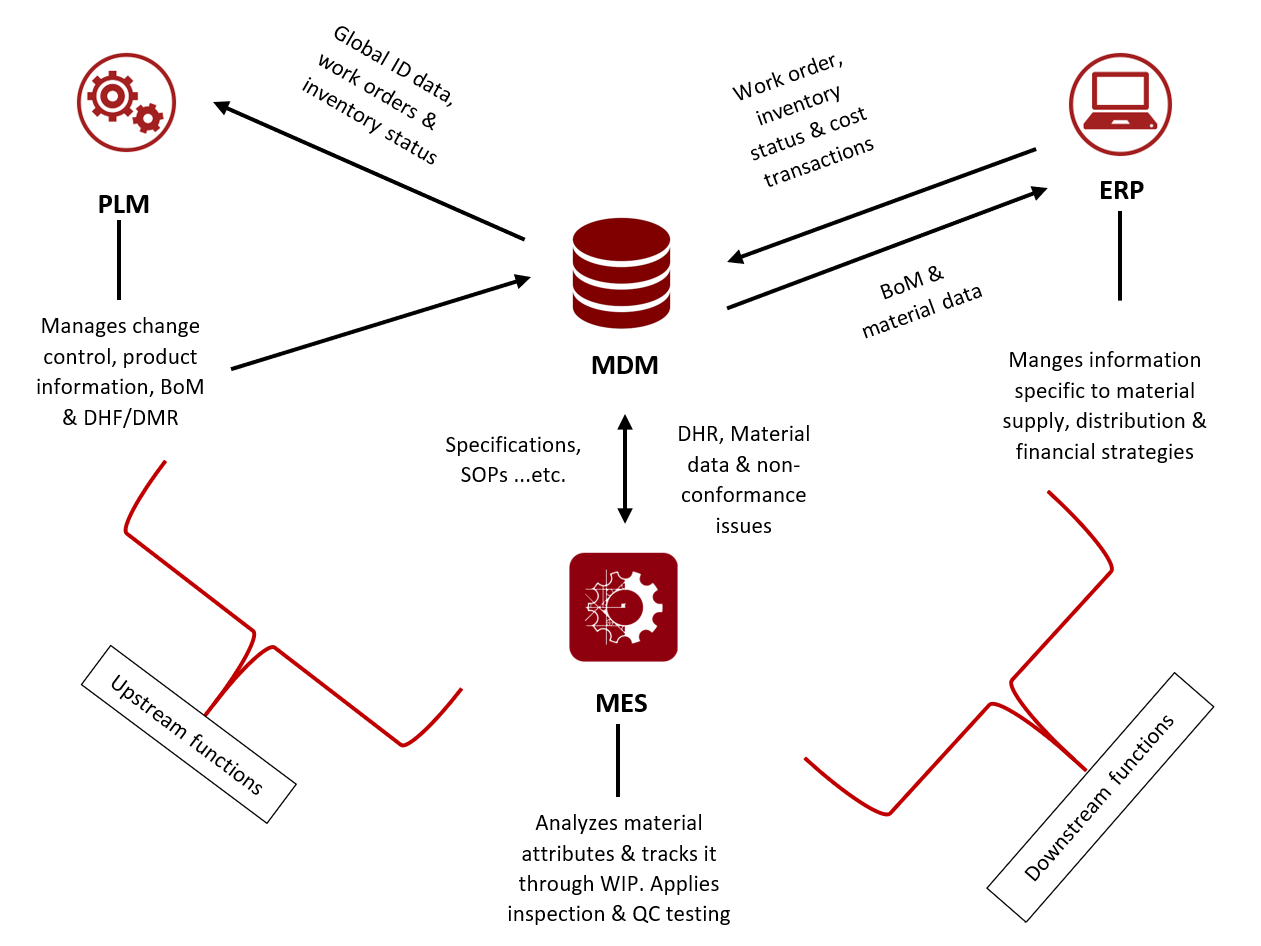
The figure above depicts one architecture for product-to-production platform. The link between PLM-ERP-MES is through a “hub-and-spoke” interface, using MDM as a mediator. The link delivers agile assembly to the production value chain. It closes the loop between downstream functions like material information, suppliers, and BoMs with upstream functions like material data, material quality, and a cost effective business strategy. It is important to mention that using MDM as a mediator is an optional model and other models should be evaluated based on the organization’s business structure and requirements.
Integrating PLM with ERP and MES systems provide visibility that will help streamline product delivery cycles, and helps recognize and correct any material quality issues before they become too costly or pose barriers to delivering a conforming product. The relationship between the three systems is a linkage of manufacturing information management to the master data management of product history and to the factory floor, providing ideal synchronization between all three systems.
[1] FDA 21 CFR Part 820.50-820.60