Leading Practices for EUDAMED and Basic UDI
Big regulatory changes are coming to the way medical device companies sell their products in the European Union (EU). Previous product management strategies are becoming antiquated with exponentially increased risk. The changing landscape demands urgency and comprehensive strategies based on informed opinions.
As a part of the changes in the EU, Unique Device Identification (UDI) and Basic UDI (BUDI) will be a requirement for medical devices. The new regulatory initiative falls under the EU Medical Device Regulation (MDR) and supersedes the Medical Device Directive (MDD), which previously had no provisions on traceability of medical devices, so the steps necessary for compliance are completely new for the EU market.
As deadlines to comply quickly approach in Spring of 2021, let’s explore the context, basics, and best practices to adapt to this new normal across the industry.
How Did We Get Here?
To better understand the context of Basic UDI, it’s important to understand the origin of UDI in the United States. UDI first came into use in 2007, when the US Congress passed legislation directing the Food and Drug Administration to develop regulations establishing the UDI system for medical devices. In an interview on July of 2013, a Senior Adviser for Patient Safety at the Center for Devices and Radiological Health stated that the underlying issue was the lack of a consistent, standardized way to identify devices. There was no explicit device identification for use in adverse event reporting or post-market surveillance. On September 24, 2013 the final FDA rule took effect in the US.
In the case of the FDA, UDI is the link that connects FDA-regulated medical device products and tracks critical quality characteristics over the entire product lifecycle. UDI aids in digital product definition and makes it possible to perform advanced analytics when scoring vendors in the FDA‘s Case for Quality initiative. There’s no doubt that the FDA UDI regulation serves as a blueprint for the EU and other evolving regulations expected to be released in China, Brazil, Australia, Japan, etc. However, each is expected to introduce new attributes according to specific intentions, and we see this in the EU.
The Basics of Basic UDI (BUDI)
In addition to implementation of their own UDI, EU MDR went one step further with Basic UDI-DI, the key identifier in EUDAMED. Basic UDI-DI is defined as “the primary identifier of a device model. It is the DI assigned at the level of the device family. It is the main key for records in the UDI database and is referenced in relevant certificates and EU declarations of conformity.” [MDR 2017/745, Annex VI, Part C]. So the DI improves the traceability of a family of medical devices over their product lifecycle.
The improved oversight will enable BUDI to act as the glue for improving safety and efficacy, making the well-being of the customer or user more transparent and the ultimate priority. For this reason, a main priority within BUDI is the identification and grouping of products. There is no stringent clarification about the size or specification of categories. A default option seems to be grouping based on already-established product families or franchises, which could result in benefits for different groupings, and more clarity around the consequences of categories. By grouping products effectively, adverse event reporting limits further harm of associated products that could have similar risks.
Practical Tips for BUDI Submissions
There are many interdependencies between other EUDAMED components and the BUDI. Based on EUDAMED requirements, a BUDI can be initiated using a Manufacturer Single Registration Number (SRN). An SRN is generated by EUDAMED once a manufacturer is registered in EUDAMED as an Economic Operator. Approved certificated information must be added to the submission package before a BUDI can be submitted for approval to EUDAMED.
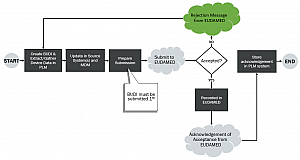
The image above represents a sample workflow for using a product lifecycle management (PLM) system to address BUDI creation, storage, and submission.
The Basic UDI-DI will not be labelled on packaging or in the supply chain; instead it is a regulatory identifier used in the Declaration of Conformity (DoC), technical documentation, Free Sales certificates, and in the Summary of Safety and Clinical Performance (SSCP).
EUDAMED will be the EU MDR-specific database, centrally collecting information about medical devices. In addition to the Basic UDI-DI, there are also the device identifier (UDI-DI) and production identifier (UDI-PI). A single Basic UDI-DI can be linked to multiple UDI-DIs, while a UDI-DI can be linked to only one Basic UDI-DI.
The Basic UDI-DI will connect relevant medical device information and provide a single source of truth for patients, healthcare professionals, notified bodies, competent authorities and the supply chain. The UDI-DI is to be included in the labelling and in the EUDAMED database. Device identifier is specific to manufacturers and device. Any lack of complete clarity requires a new unique UDI-DI. In addition to the UDI-DI, the UDI-PI is to be included in the labeling and if applicable, in the implant card. Production identifiers identify unit of device production.
The Broader Initiative for Regulatory Information Management
Since tech doc submissions and certificate management is also a critical part of EU MDR, Regulatory Information Management (RIM) systems and processes have also become critical to efficiency in compliance towards EUDAMED. RIM systems manage regulatory data and documents throughout the product lifecycle. RIM is more focused on technical documentation than BUDI, and BUDI does need to be referenced across UDI-DI, product registrations, technical documents/certifications/DoC, the SSCP, or in vigilance and post-market surveillance. Therefore, BUDI should be registered as a precursor to all the other processes. BUDI has its own submissions and lifecycle that trigger submission to EUDAMED.
Companies that maintain their product data in a PLM system, and who may have associations that represent product families, should implement a workflow process that can be kicked off to start the BUDI request procedure. As an alternative and a part of the device identification process, a regulatory team can identify and associate a BUDI to a product, and then trigger a workflow for approval and subsequent submission.
The following images show solution architecture and product master data infrastructure that directly address submissions to EUDAMED by strengthening initiatives around data management.
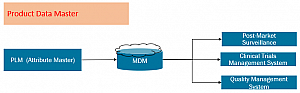
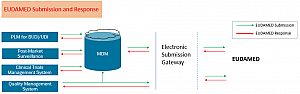
The Bottom Line
As a guiding document, the final regulations will be subject to the interpretation of the Court of Justice of the European Union. With so much at stake and a lack of precedent, organizations must focus on risk mitigation through improved processes.
The question then becomes around the alignment of this BUDI process in Europe. EUDAMED requires about 50 attributes in approved submissions for the product family BUDI registration listing storage and approval; are these submissions going to be unique?
Soon there could be hundreds of attributes required for an international organization. Already, we expect 350+ new UDI attributes to be required (future and present) with the six pending UDI initiatives in 28 EU countries.
To address the growing challenges of regulatory warnings while maintaining profitability and global competitiveness, medical device manufacturers are leveraging a variety of initiatives. These include a focus on the FDA case for quality, strategic integration of current and planned systems to normalize metadata, and efficiently and effectively tracking worldwide product submissions and registrations (with RIM). For all of these initiatives, a strong PLM backbone improves success, and opens opportunity for future initiatives including digital app connectors, machine learning and predictive analytics.
More Reading
eBook: The Imperative of Better RIM



