How PLI Can Decrease the Cost of Quality and Compliance
Early adopters of PLM have achieved valuable benefits to the product development process. However, PLM alone can no longer help.
WHAT IS PLI?
Product Lifecycle Intelligence (PLI) is an application of advanced analytics across the product lifecycle used to improve innovation results.
WHAT DOES IT DO?
PLI applies machine learning techniques to mine operational insights from product development data in business systems including product lifecycle management (PLM), enterprise resource planning (ERP), quality management systems (QMS), manufacturing execution system (MES) and more.
WHAT ARE THE BENEFITS?
PLI helps organizations predict the impact of product development decisions on key business performance metrics, like demand, cycle time, cost, quality, regulatory compliance, manufacturability and supply chain efficiency.
PLI helps innovators prescribe evidence-based recommendations to improve future innovation outcomes.
The insights gleaned from PLI benefit multiple business contexts, including R&D, regulatory,
finance, quality, manufacturing, supply chain and service.
Between the EU MDR, the FDA’s Case for Quality program, and new UDI attribute requirements, the global regulatory landscape for medical devices is progressing with an emphasis on product quality and post-market surveillance. As companies re-evaluate their current position, they have an opportunity to take a data-driven approach to R&D, quality and regulatory. Companies can improve the quality of their product portfolios while streamlining their compliance journeys by integrating product quality and compliance systems and deploying digital technologies such as Product Lifecycle Intelligence.
Building the backbone:
A common theme with medical device companies is the existence of multiple siloed legacy quality systems within the organization. Studies have shown that achieving true interoperability between their CAD, PLM, and QMS systems can decrease internal and external failures by up to 50%1. The ability to define critical to quality characteristics during the early stages of product development, create risk-based control plans, and close the loop on quality feedback will set top-performing companies apart from competitors in the next three to five years.
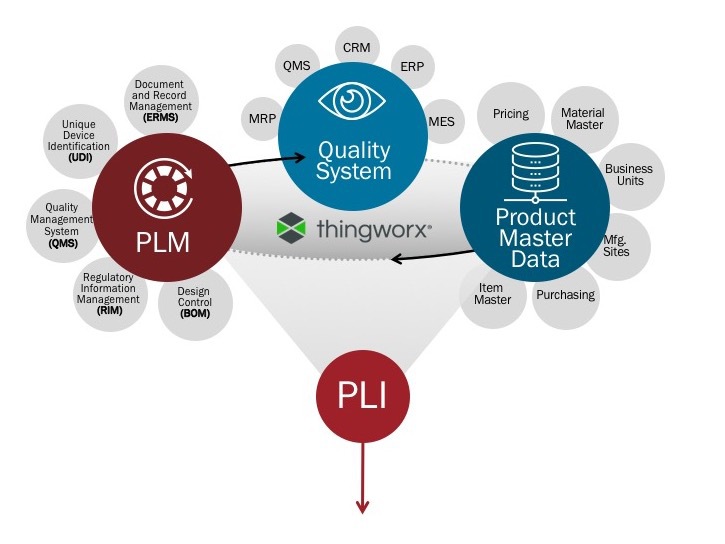
Harmonizing PLM and QMS is the first step in closing the quality loop. The capability to incorporate corrective and preventative action (CAPA), complaints, and non-conformance data into the design process begins the shift from corrective to preventative, reducing the cost of poor quality. Further integration with regulatory management functionality completes the “backbone” of product quality and regulatory compliance. Product quality integrated with product compliance expedites the regulatory submission process and provides traceability to compliance across multiple regulatory jurisdictions. The regulatory documentation data package no longer lags engineering innovation but instead becomes a direct output of the design process. PLI connects the dots between the newly consolidated systems as well as the multitude of siloed legacy systems.
Connecting the dots:
Early PLI technologies have been employed in the medical device industry for the past 15 years by tracking signature failures in products using algorithms. Companies are able to place watchdogs for the exact data patterns captured by PLI to prevent specific quality issues.
The next stage of PLI can be used to capture quality systems data across ERP, PLM, MES, master data management (MDM), etc. to generate data failure patterns across systems, expanding failure detection capabilities. This also aids companies in identifying root causes of failures in a shorter time frame to contain quality issues and reduce recalls by being nimble with their quality data.
Beyond detection and root cause analysis, PLI has additional use in monitoring post market surveillance data and field data from censors embedded in devices. Detected failures may be defined and acted upon. PLI can define the harms, hazards, and risk priority numbers on the actual product that is managed in PLM which is directly associated with a 3D CAD model. As soon as failures occur, service technicians can be deployed, and the criticality of the failure can be readily assessed. For example, in the case of a critical failure on high risk products, regulatory reports can be autogenerated and corrective actions prioritized, reducing the chance of a warning letter for late regulatory reporting.
Medical device companies can use PLI to help navigate their current compliance journeys and create a sustainable set of solutions to rapidly assess and prepare their product portfolios for future regulations. When coupled with the consolidated product quality and compliance systems, PLI will push companies from corrective to preventative actions, reduce the cost of poor quality, and expedite new product introductions across multiple regulatory jurisdictions.
The FDA Case for Quality is a blueprint for evolving worldwide regulatory changes such as the EU MDR. By utilizing key performance indicator based algorithms which were created by the Case for Quality, companies can assess the health of their organizations, help remediate warning letters, and, ideally, prevent quality escapes from happening in the first place. Regardless of existing or future systems, PLI can help organizations generate value by utilizing new and historical data more effectively.

