Digital Thread in Life Sciences: The Future of Product Innovation
Our world changes daily in front of our eyes. The COVID-19 pandemic continues to have profound effects on our lives. As we move into a new future, one thing is for certain... the future will be increasingly digital.
The Shifting Landscape in Medical Device
It’s clear that pandemic-related challenges will drive lasting changes in the way we all work, especially within industries that are directly affected by the pandemic.
Imagine Susan, a 43-year-old mother of two. She has worked in the medical device industry since 2002 and has experience in various stages of the product lifecycle. Currently, she serves in a product management role and has spent the last two years developing a plan for the next big product for her (fictional) $2.2 Billion medical device company, Kuality Kare.

In March of 2020, the world she knew was turned upside down, and her focus became firefighting through a situation she had not yet had a chance to process. There were a million questions she didn’t have the right answer to, and no textbook example to hold up as a leading practice.
Susan’s story is not unique. Companies across the medical device industry were disproportionately impacted due to the pandemic. Early in 2020, as healthcare organizations initially braced themselves for overcapacity, the industry struggled to keep up with the rising demand for essential products while also struggling to adapt to the implications of halting elective procedures. The industry reeled as workforces became remote overnight. For many, protecting employees came at the expense of effective collaboration.
This not only impacted product development and manufacturing, but sales, training, and product servicing, as much of the industry was underprepared to solve challenges traditionally resolved in person in this new remote working environment.
It is clear that the companies who focused on building their digital capabilities in recent years have had a significant advantage in adapting and responding to the pandemic, but what does this signal for the future?
According to the Harvard Business Review, in 1958, the average lifespan of companies listed in Standard & Poor’s 500 was 61 years. By the 1980s it was down to 25 years. Today it is less than 18 years. The reason for the sharp decline? The rate of change in the world has increased dramatically due to digital transformation. Life Sciences organizations must embrace digital transformation if they are to survive in this rapidly changing world.
Redefined Possibilities: The Digital Thread and the Digital Twin
So why do some companies fail to embrace a digital world while others thrive? The organic evolution of processes and technologies has resulted in a disjointed experience within the product lifecycle, from discover to create to make to sell. The solution is the digital thread.
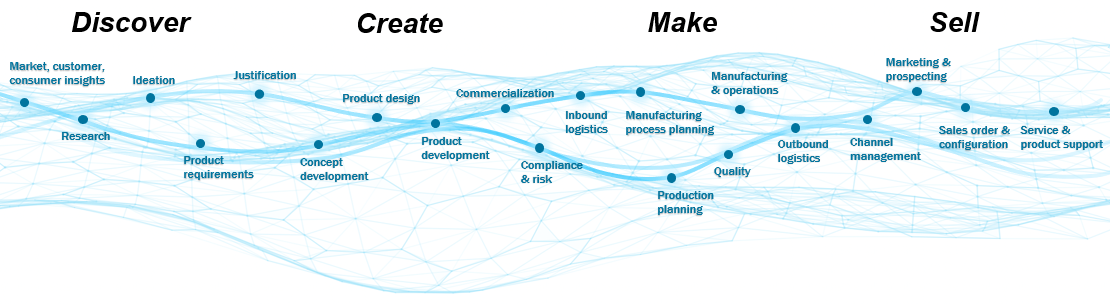
If the value chain represents all the activities that a company completes to deliver a product to the market, then the digital thread is the fabric that holds it together. The stronger the fabric, the stronger the value chain. The focal point of the digital thread is the digital twin, or the virtual representation of the physical product. The digital twin can be used in design, testing, monitoring, servicing, and other functional areas to augment product management capabilities.
Reimagining Product Innovation with the Digital Thread
To examine how the digital thread enables innovation in the face of great challenges, let’s jump back to the present and check back in with Susan and her colleagues at our fictional medical device company, Kuality Kare. In this scenario, we assume they have a modern digital infrastructure in place, and this dramatically improves their ability to innovate and deliver.
Using advanced analytics, Kuality Kare has identified a market need brought about by the pandemic. With minor changes to an existing product, they can help fill this crucial gap. If Kuality Kare can act quickly, it can help the world combat the pandemic and serve shareholders by offsetting lost revenue to their elective surgery products business. Luckily, the company has spent the last few years investing in its digital capabilities, so Susan feels confident in her team’s agility and preparedness for this challenge.

Susan’s colleague Bill is driving new ways of working in product design. He recently led Kuality Kare’s shift to cloud-based CAD tools – which integrate seamlessly with their product lifecycle management (PLM) systems – and Bill is especially grateful to avoid the pains that come with traditional file-based CAD systems that limit collaboration. His rapid adoption of game-changing new features – like the ability for his design team to collaborate on the same CAD model in real time – allows them to iterate quickly as they adjust an existing product’s design to fill the identified market need.
This CAD model is the basis for the digital twin – a concept that Susan is pushing at Kuality Kare. Digital twins come in a variety of forms, often incorporating several discrete models threaded together. These representative models can be composed to work together, allowing organizations to simulate an object’s behavior in a specified digital environment, subject to a known set of assumptions and conditions. Applying physics-based digital simulations feed incredible insights to product design that do not require the time and expense of physical prototype creation and testing.

Susan’s intern Rachel has set up a demo to show the team how their 3D CAD models can easily be loaded into an augmented reality application, further expanding the use of the digital twin. Rachel explains how this would allow for a virtual product review that leverages the digital twin for an accurate and informed session in which reviewers need only their phone to participate. These models can then be leveraged again later in the product lifecycle to help with sales, training, and servicing.
Key Elements of the Digital Thread
Because Kuality Kare is quite advanced in investing in digital transformation, leaders like Susan know that the strong digital infrastructure will help her team quickly get this new product to market and support patients in crisis.
The pandemic also proved as a testing ground to validate the processes and technologies they have invested in. Today, Susan can reflect upon some key elements for success as her team prepares to release their next product.
Product Development
- Foundational PLM Systems – These foundational systems manage product data across the lifecycle. In Susan’s situation, PLM enables their product concept to move into production through an efficient engineering change management process, designed to provide the right information to the right people at the right time. As the product moves to production, sourcing managers can access the BOM in order to communicate effectively with suppliers and ensure that the right materials arrive where and when they are needed. Simultaneously, manufacturing engineers will use the digital twin along with factory simulation tools to prepare for manufacturing at scale.
- Extended Reality – Sales professionals may not be able to enter the hospital, but they can provide a virtual demonstration of the product leveraging the digital twin and augmented reality, so that their customers can assess the product’s real-world capabilities. These models may also be used to train healthcare workers in the field without requiring an in-person training session.
- Servicing Excellence – When a nurse realizes that a device is not working properly, it disrupts patient care and needs to be fixed ASAP to treat critical patients. But there are challenges. Devices are in short supply. There’s no time to send the product into a servicing center, and they cannot bring in an outside technician. With a digital thread in place, a nurse simply scans a code on the product to connect virtually to a remote technician. The technician diagnoses the problem based on feedback from IoT sensors in the device and walks the nurse through a simple repair using directions relayed in an augmented reality app on an iPad.
- Smart Connected Products – Kuality Kare can run predictive analytics on data from smart devices and alert technicians of devices that are at risk of malfunction before critical issues take them offline. This allows technicians to proactively service the product and reduce downtime. This is made possible by sensors placed in every device that transmit data back to Kuality Kare’s IoT Management System. The data collected can be analyzed to uncover patterns and trends, identifying scenarios that accurately predict the next time a product will require proactive servicing.
Manufacturing
Susan has prioritized critical aspects of the digital thread important to her business (and her product development focus) at this time, but she understands that there is more work ahead in Kuality Kare’s digital transformation journey.
For the next phase, Susan has the goal of eliminating paper-based tracking systems on the factory floor and has the following digital capabilities on her radar:
- Manufacturing Execution System – The MES is the solution that will help Susan’s company digitally manage the production process. It connects, monitors, and controls all physical systems in place for the manufacturing process. An important output of this system will be the Device History Record that proves Kuality Kare correctly applied quality controls throughout production and conforms to regulation 21-CFR-820.
- Industrial IOT – Working with manufacturers to enable IIOT can help Kuality Kare 'get smart' about the manufacturing process. With sensorization, Kuality Kare can monitor the production of equipment and find ideal operating conditions to increase production yield. Other opportunities include optimizing energy consumption at the asset level and using edge analytics to improve quality control.
One thing is certain to Susan – the many capabilities the digital thread provides to Kuality Kare add up to a sizable advantage that will help the company manage the current crisis while building more resiliency as they prepare for the future.
The Prescription for Digital Thread Success
Unanticipated disruptive events like COVID-19 have made the digital thread more important than ever. The challenges faced as a result of the pandemic serve as a call to action for the life sciences industry to invest in its future, but the path forward is still not clear.
How can organizations move from their deeply entrenched legacy systems to a state-of-the-art digital ecosystem? More importantly, how can they train their people to embrace and make use of these tools?
Here are some basic tips for implementing the digital thread and digital twin successfully.
- Don’t worry about perfecting the digital transformation roadmap. Instead, we recommend a simultaneous, dual-pathway approach. On the first path, define an overall direction for where you want to go, with a high-level view of systems and connections. Consider separate roadmaps for aspects of digital transformation, like data management, smart connected factories, and smart connected products. Revisit and adjust every 6-12 months based on learnings or business changes. The second path should focus on tackling important current business problems leveraging new and emerging technology. This extremely agile path puts out current fires while not compromising longer-term planning.
- Don’t focus on technology (and proofs of concept that demonstrate technology). Focus instead on creating digital proof points with measurable business value. Think about the application of the technology, and the purpose and value of solving a particular business challenge to drive big impact. For example, don’t demonstrate augmented or extended reality technology. Instead, demonstrate the ability to operate a surgical robot remotely or to conduct training remotely with the goal of limiting physical presence in hospitals during a global pandemic.
- With an initial digital strategy and proof points established, next define the capabilities the organization needs to have the greatest business impact. This is a combination of people, process and technology that will support an actual digital transformation that is sustainable and scalable, no matter what the future brings.
Susan has done a lot of this work already. Her next step will be to seek ways to extend and build value across the value chain – both within Kuality Kare and to the value chains of suppliers, distributors, and customers. Although Susan’s story is fictional, companies that follow her example will reap the benefits now and position themselves to continue to outpace the competition.



