Navigating Medical Device Industry Trends by Adopting the Digital Thread
Welcome to our new blog series where we highlight the most impactful trends in the medical device industry across four dimensions: technology, business, patient care and compliance. Each post will address how the digital thread can help companies embrace these trends to improve business outcomes.
Blog 1: Navigating Medical Device Industry Trends by Adopting the Digital Thread
Blog 2: Spotlight on Technology: Adoption of Artificial Intelligence in Medical Devices
Blog 3: Technology Trends Continued: The Growth of Connected Devices and the Internet of Medical Things
Blog 4: Medical Robotics: The Future is Now
Blog 5: AI's Growing Influence on the Medical Device Company’s Value Chain
The pace of change in the medical device industry is both exciting and extraordinary.
Major market forces, such as intensifying regulatory scrutiny, emerging digital technologies, especially Artificial Intelligence and Internet of Medical Things, a shift to services-focused product lifecycles, supply chain resilience, new cyber security regulations, a shift in quality system regulations, and more are causing core paradigm shifts in business strategies and processes alike.
The medical device market is expected to surpass $600 Billion by 2025. This growth is supported heavily by demographics and new technology breakthroughs. It’s also increased by the advancement of artificial intelligence, as well as the Internet of Medical Things (IoMT), extended reality, scalable cloud computing and technologies that are accelerating the development of digital twins.
Companies need to brace for a rapidly evolving future and invest now. There are now well over 100 medical device companies (including divisions embedded within Pharma) with revenues in the billions, but not all companies are truly making the investments necessary to compete in the near future.
At the same time, we see most of the mega-sized tech companies making inroads in the medical technology, diagnostics and healthcare space. These companies are uniquely positioned with their super-sized AI models, massive capital, unprecedented talent (some from medical acquisitions) and incredible compute power to make significant dents in the industry.
Discovering the Digital Thread
Medical device companies must see digital thread investment powered by artificial intelligence (AI) models as their number one strategic imperative or risk falling behind. The digital thread refers to the seamless flow of data and information across different stages of a product's life cycle. This includes design, manufacturing, use and service, creating an integrated and connected data ecosystem.
Adoption of the digital thread will create opportunities for medical device companies to better serve more people around the world with increasingly innovative and affordable diagnostics, treatments and combination devices.
Benefits of the digital thread include:
- Faster development cycle time
- New product-driven growth
- Enablement of business scaling
- Streamlined regulatory compliance
- Reduced business and clinical risk
- Simplified product registrations
- Improved asset utilization and reduced downtime
- Reduced scrap and waste in prototyping and manufacturing
- Better energy / reduced environmental footprint
- Reduced manufacturing and field service costs
- Closed-loop quality management
- Improved insights from connected products
The medical technology industry has unique processes and requirements that span the value chain. These include research, design, quality and product engineering, clinical trials, validation, pre-market approval, production, post-market support and product obsolescence. Increased regulatory pressures and value-based healthcare are shifting strategic priorities, however, the digital thread is transforming product development, manufacturing, clinical processes and real-world care (as well as evidence capture). Adopting the digital thread throughout the value chain is essential for scaling organizations in response to industry growth. It enables companies to proactively address or leverage disruptive forces more effectively.
The following figure illustrates the digital thread concept for medical device companies. This is just representative and not all inclusive of the dozens of processes and capabilities that span the entire product lifecycle. It is worth noting how the digital thread spans the Total Product Lifecycle, which is a key FDA concept and expectation for many aspects of managing data within the quality system.
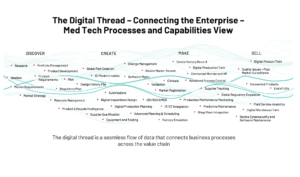
TPLC and the Digital Thread
The FDA mandates a Total Product Lifecycle Approach (TPLC). TPLC expects manufacturers to integrate pre and post-market activities into a holistic view to help ensure device safety and effectiveness from design through commercialization. The digital thread mirrors and expands upon this concept by integrating engineering, regulatory, manufacturing quality and service processes into a more seamless flow of information, which is then enhanced by data extrapolation and analytics.
Stay tuned for our next blog where we’ll tackle technology and why companies need to develop and implement a robust digital thread strategy.

