We help companies unify quality processes and data across the product lifecycle with a digital QMS, fostering a culture of quality that ensures the delivery of more effective, safer products that improve patient lives.
As the medical world continues to shift toward value-based healthcare, the need for product quality and efficacy improvements, together with cost containment, is becoming more pressing. For decades, maintaining Food and Drug Administration (FDA) Part 820 compliance and ISO 13485 certification has been the top priority for medical device manufacturers, but it has not always delivered product quality and patient safety, as signaled by weaknesses identified in FDA warning letters.
Simply focusing on maintaining compliance is not enough, not only in the U.S. but globally.
The Case for Quality Imperative
The FDA’s CFR Part 820 compliance requirements act as a blueprint for worldwide regulatory compliance guidance, as noted by the European Union (EU) Medical Device Regulation (MDR). Quality and regulatory leaders assure their engineering and quality departments are staffed and resourced appropriately, and there are procedures in place to resolve and report issues. Most confirm that when new regulations arise, there are people and policies in place to adjust the processes to maintain compliance. But, the cost of making these changes is high and the effectiveness of maintaining product quality is debatable.
To change the mindset of the medical device industry from ensuring compliance to enhancing product quality and patient outcomes, the FDA started the Case for Quality (CfQ) initiative, which recommends manufacturers focus more time up-front on quality by design versus pure compliance.
85% of respondents indicated that the primary role of quality is compliance. Only 10% said it is to drive product quality and process improvement.
Building a Culture of Quality and Innovation, An Axendia Medical Device Industry Survey
You might be asking…
How do I go beyond compliance and start building quality at the on-set of design?
How do I include complaints and nonconformances within new product development workstreams?
How do I provide proof of an engineering change impact analysis in a required regulatory report?
Digital Quality Management System (QMS)
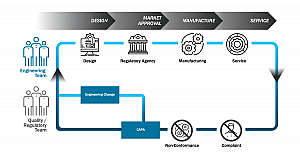
A digital QMS connects and harmonizes the data between foundational processes like design control, document control, change, nonconformance, complaint, CAPA and audit management over the product lifecycle. It provides product development teams visibility to lessons learned, facilitating quality by design in new product development and streamlining the resolution of engineering change orders.
While it is common for medical device manufacturers to have point solutions for quality and compliance management, many lack integration across them and to the product data record. Implementing a digital QMS increases data transparency and helps enable a foundational culture of quality.
Business Value Benefits
- Up to 40% lower cost of quality
- 65% fewer critical engineering change orders
- 95% lower nonconformance resolution times
- 72% greater CAPA effectiveness
- Increased enterprise-wide quality visibility
- Improved product safety and efficacy
- Greater customer loyalty
- On-time regulatory reporting
Sources: Closed-Loop Quality Management: Integrating PLM and Quality Management, Aberdeen Group; Quality Management Survey, LNS Research; The Quality Auditor Primer, Quality Council of Indiana
Building Up Digital QMS Maturity

Basic
- Multiple, disconnected quality management systems
- Mix of paper product data management and basic PLM
- No analytics capabilities
- High cost of poor quality
- Poor customer satisfaction
- Limited visibility of quality information across functions
- Minimal traceability of adverse events and service issues
- Reactive risk management
- Manual data transfer errors
- Uninformed decisions

Formal
- Mix of consolidated and siloed quality management solutions
- Formal PLM with foundational processes (document and design control, change management)
- Analytics capabilities limited to descriptive and diagnostic
- Average cost of poor quality
- Fair customer satisfaction
- Limited visibility of quality information across functions
- Basic traceability of adverse events and service issues
- Contingent risk management
- Some data transfer errors
- Better informed decisions

Connected Closed Loop
- Greater integration of foundational PLM with quality management processes and data
- A few siloed quality management and foundational processes
- Improved cross-functional visibility of enterprise quality data
- Predictive and prescriptive insights
- Elementary quality by design
- Developing culture of quality with focus on lower cost of poor quality
- Good customer satisfaction
- Intermediate traceability of adverse events and service issues
- Proactive risk management
- Minimal data transfer errors
- Well-informed decisions

World Class Predictive
- Harmonized consolidation of foundational product management with quality processes and data
- Enterprise-wide quality data visibility
- Predictive insights using AI and ML for future product development, quality optimization, preventive risk management and control verification
- Optimized quality by design
- Mature culture of quality focused on the cost of good quality
- High customer satisfaction and loyalty
- Real-time traceability of adverse events and service issues
- Six sigma-controlled data errors
- Automated, real-time change impact analysis, regulatory reporting, adverse event automation and closed loop feedback of product and process performance
How We Help Clients Enable Digital QMS
Digital QMS success requires a phased approach to consolidating, connecting, and harmonizing product and quality processes and data to enable cross-functional team sharing and a culture of quality. Kalypso can help you assess the current state of your organization, align on the key value drivers and develop a value-based roadmap for improvement using our digital QMS maturity model.
Our strategic three-phased approach is designed to drive maximum value with an iterative crawl-walk-run cycle.

Assessment and Strategy
- Perform an assessment to understand current state quality management processes, system infrastructure, organization maturity, regulatory initiatives and challenges
- Review and refine quality and compliance goals
- Develop a digital QMS improvement roadmap, prioritizing objectives, IT investments and organizational changes

Proof of Value
- Implement a small-scale proof of value deployment at a subset of business units or manufacturing sites
- Gain feedback and measure results from the use cases included in the proof of value
- Refine the planned system architecture and recommended quality practices for scalability across other business units and manufacturing sites

Scale Across the Organization
- Develop an enterprise-wide digital QMS roll-out plan, incorporating necessary process, system architecture and organizational changes to achieve the desired results
- Plan for multiple use case roll-outs to scale fully and achieve world class predictive QMS maturity
- Continuously review progress, report on metrics and communicate value to the organization to drive culture of quality
Our Work in Digital QMS
Case Study: Medical Device Manufacturer
Transformed product development and quality data management with a digital QMS solution, optimizing product registrations, improving product quality and accelerating time to market
Context
- Organization needed a better way of tracking worldwide product registrations
- Research & Development and engineering functions needed to improve quality data exchange
- Management wanted to use digital transformation to foster a new culture of quality
- Kalypso performed an assessment of the existing QMS processes and infrastructure and helped the organization define an improvement roadmap
Approach
- Created and launched a change management program and governance structure for the new Digital QMS initiative
- Conducted workshops to define requirements
- Designed, configured and validated the software
- Deployed the solution globally via a phased rollout and a go-live support model
- Monitored user adoption and provided organizational change management to ensure solution adoption
Results
- Harmonized quality assurance, regulatory assurance, research and development, procurement and manufacturing processes
- Reduced quality issue resolution times and improved accuracy of root cause analysis
- Accelerated global product submissions and registrations
- Enhanced decision making when designing new products and prioritizing CAPAs
- Improved effectiveness of management reviews