Fixing Siloed Design & Manufacturing Processes in the Medical Device Industry
Are the designs you're meticulously crafting translating seamlessly into the manufactured products at your company? It's a common challenge faced by many businesses, where the envisioned design often encounters hurdles during the manufacturing phase, prompting blame on perceived incompetency within the organization. These discrepancies stem from various sources, including the inadvertent use of outdated component versions in new designs, reliance on obsolete manufacturing equipment or processes and mismatches between parts and the design's bill of materials (BOM).
This misalignment can result in a cascade of issues, ranging from quality defects, malfunctions, and performance inconsistencies to potential product recalls. This is especially concerning for medical device companies.
Manufacturing Process Management (MPM) offers tailored value propositions that align with the unique requirements and challenges of the healthcare industry that we’ll discuss later.
Consequently, it can also lead to production delays, cost overruns, and ultimately, customer dissatisfaction. Furthermore, such lapses can erode the company's market reputation, highlighting the critical need for effective coordination between design and manufacturing teams, investment in modern technologies, and meticulous supply chain management to bridge this gap and ensure the seamless realization of design intent into the final product.
How MPM Addresses This
If you’re encountering challenges when aligning design and manufacturing processes, a manufacturing process management (MPM) platform may help to solve them. These platforms can foster seamless communication and collaboration between design and manufacturing teams and ensure accurate alignment between design specifications and manufacturing requirements, reducing the risk of discrepancies in component selection.
With detailed process planning functionality, organizations can optimize manufacturing processes to align with design specifications, improving efficiency and quality.
Furthermore, version control features ensure that teams work with the most current information, mitigating the risk of errors and enhancing overall product quality. Through these capabilities, organizations can bridge the gap between design and manufacturing, resulting in smoother production processes, higher product quality, and increased customer satisfaction.
Value Propositions of the Medical Devices Industry and MPM
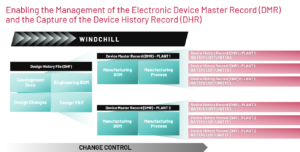
For medical device companies, Manufacturing Process Management (MPM) offers tailored value propositions that align with the unique requirements and challenges of the healthcare industry:
- Compliance and Regulatory Adherence: MPM ensures strict adherence to regulatory standards and compliance requirements specific to the healthcare sector, such as FDA regulations (e.g., 21 CFR Part 820). By providing robust documentation, and audit trails, MPM helps medical device companies navigate complex regulatory landscapes and maintain compliance throughout the manufacturing process.
- Patient Safety and Quality Assurance: MPM prioritizes patient safety and product quality, critical aspects of medical device manufacturing. By enforcing standardized processes, rigorous quality controls, and real-time monitoring, MPM helps ensure that medical devices meet stringent safety and efficacy standards, ultimately enhancing patient outcomes and reducing the risk of adverse events.
- Efficiency and Cost Reduction: MPM optimizes manufacturing processes to improve efficiency and reduce production costs without compromising quality or safety. By automating workflows and optimizing resource utilization, MPM enables medical device companies to achieve operational efficiencies and cost savings while maintaining high standards of product quality and compliance.
- Agility and Innovation: Agility and innovation within medical device companies is achieved by increasing product and process design degrees of freedom, one intended design accommodated by multiple plant and line-specific variations. By facilitating rapid prototyping, flexible production scheduling, and efficient change management processes, companies can avoid repeating old mistakes and inefficiencies. MPM helps medical device companies stay competitive in dynamic healthcare markets and respond quickly to emerging customer needs and market trends.
- Risk Management and Traceability: MPM provides comprehensive risk management capabilities, allowing medical device companies to identify, assess, and mitigate risks throughout the manufacturing process. By ensuring traceability of materials, components, and processes, MPM enhances product safety and facilitates timely recalls or corrective actions in the event of quality issues or regulatory non-compliance.
- Customer Satisfaction and Brand Reputation: Ultimately, MPM contributes to enhanced customer satisfaction and brand reputation by delivering high-quality, safe, and reliable medical devices that meet or exceed customer expectations. By consistently delivering products that inspire trust and confidence among healthcare professionals and patients, MPM helps medical device companies build strong brand loyalty and differentiate themselves in competitive markets.
MPM offers medical device companies a comprehensive suite of value propositions tailored to the unique requirements of the healthcare industry, including regulatory compliance, patient safety, efficiency, innovation, risk management, and customer satisfaction. By leveraging MPM solutions, medical device companies can optimize their manufacturing processes, deliver superior products, and drive business success in the healthcare market.


